On June 19, 2023, MIDAS signed a Memorandum of Understanding (MOU) to collaborate on the prioritization and assessment of technologies to support the research and development of health innovations in Thailand.
Thailand’s medical innovations have very high potential. As a nation, we are evolving from being the end-users of innovations as patients to being ones that integrate and implement these advancements throughout our healthcare system from infrastructure to management to treatment systems. The journey of medical innovation now goes beyond just medicines and doctors, with innovators actively developing health and medical technologies which can transform all aspects of the healthcare system.
The demand for medical innovations is soaring, resulting in a highly competitive market. It has also become more feasible to integrate innovations into the healthcare system, leading to an increased volume of innovation development at both the industry and academic levels. To harness this potential, boosting Thailand’s economy through medical innovations and improving the healthcare system, it is crucial to evaluate their practical feasibility, particularly at the research and development stage.
The Medical Innovation Development and Assessment Support (MIDAS) under HITAP (Health Intervention and Technology Assessment Program) is dedicated to establishing a robust and efficient medical innovation ecosystem in Thailand. MIDAS aims to push innovations from innovators into the national health system, eventually integrating them into the benefit packages of the National Health Security Office’s Universal Coverage Benefit Package (UCBP), commonly known as the “Gold Card” benefit.

To achieve this, Dr. Teerawat Wiwatpanit, a research program manager and a research fellow at MIDAS, explained that their role involves evaluating medical innovations during the research and development stages. This ensures that these innovations can address the needs of Thailand’s healthcare system and ultimately benefit the people. A critical aspect of this process is evaluating health and medical innovations that have not yet gained market access. This period provides a valuable opportunity to refine the innovation to better align with the needs of healthcare system and market in Thailand.
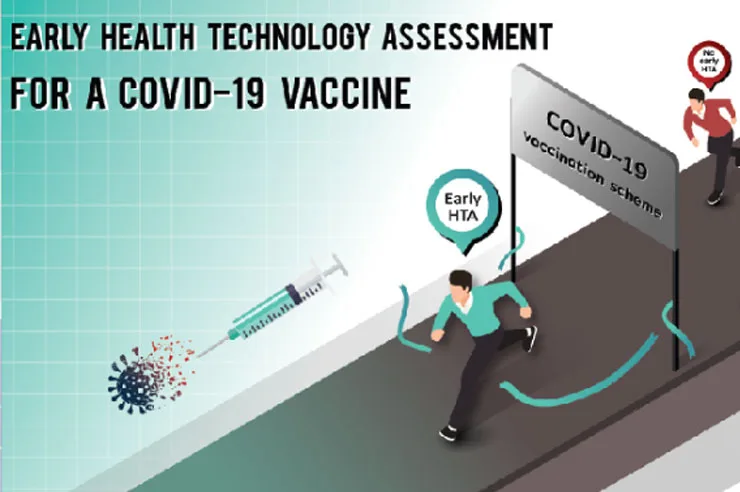
Despite the growing number of medical innovations in Thailand, many are still in development and have not reached manufacturing stage yet. As a result, Thailand continues importing a significant volume of medical products each year, including medicines, vaccines and medical devices. This reliance in imports is partly due to a lack of guidance for innovators on how to develop products that meets the healthcare system needs—guidance that can be obtained through Health Technology Assessment (HTA) and, particularly, early HTA research.
Early HTA involves an evaluation of medical innovation at the early research and development stages to assess the potential, quality, and cost-effectiveness. The insighted gained from early HTA, including the impact of the innovations on public health systems, hospitals, and daily life, are invaluable in guiding the innovators throughout their development process.
Established in mid-2023, MIDAS aims to assess and prioritize health innovations in development, identifying the most promising strategies to advance these innovations.
Dr. Teerawat added that MIDAS’s short-term goal is to collaborate with innovators to define desired characteristics of technologies (target product profiles) that can contribute to their future success from both clinical and economic perspectives. The medium-term goal is to prioritize innovations that address the needs of Thailand’s healthcare system and guide public and private research support agencies in investing in high-potential innovations.
Looking ahead, MIDAS aims to build a network of medical innovators and become a platform connecting all the actors in the medical innovation ecosystem including innovators, patients, healthcare providers, policymakers, and funders. This approach will support high-quality research that meets healthcare needs and facilitate their implementation under the “Gold Card” universal health benefit package, thereby improving the efficiency of the innovation ecosystem and increasing the public’s accessibility to high-potential medical innovations.
Currently, MIDAS is working on four early HTA projects for medical innovations, with two more in the pipeline, and one academic project on identifying factors affecting the feasibility and success of medical innovation development. Innovation development takes time, and the early-stage assessment or early HTA can help shorten this process significantly while ensuring innovations meet future success criteria, leading to faster registration and reimbursement evaluation processes.
MIDAS’s work are aligned with Thailand’s goal of becoming a regional medical hub by identifying and evaluating high-quality and cost-effective medical innovations. This initiative will ultimately strengthen Thailand’s independence in the supply of medical products, reducing reliance on imports and boosting the economy by addressing the healthcare and public needs.
To read in Thai, visit: https://www.thecoverage.info/news/content/7099
Authors: Serah Clarence, Pattranit Pohnatchariyagul, Teerawat Wiwatpanit